Available since 2015, the treatment of patients with metastatic castration-resistant prostate cancer (mCRPCC) with the beta-emitter Lutetium-177 has had several early-stage dosimetry studies published, demonstrated promising results in phase 2, and recently the first phase 3 trial has ended recruitment (Vision of Endocyte/Novartis).
Science is making strides and nuclear medicine is gaining more candidates. 225Ac-PSMA or 213Bi-PSMA targeted alpha therapies (TAT) have some dosimetry preliminary and some retrospective observational studies. These first clinical experiments with 225Ac-PSMA demonstrate promising antitumor activity with a psa response rate>50% of 63% -70%, and a response duration of 10-15 months, in addition to complete remissions in approximately 10% of patients, some of them with persistent relapse-free survival.
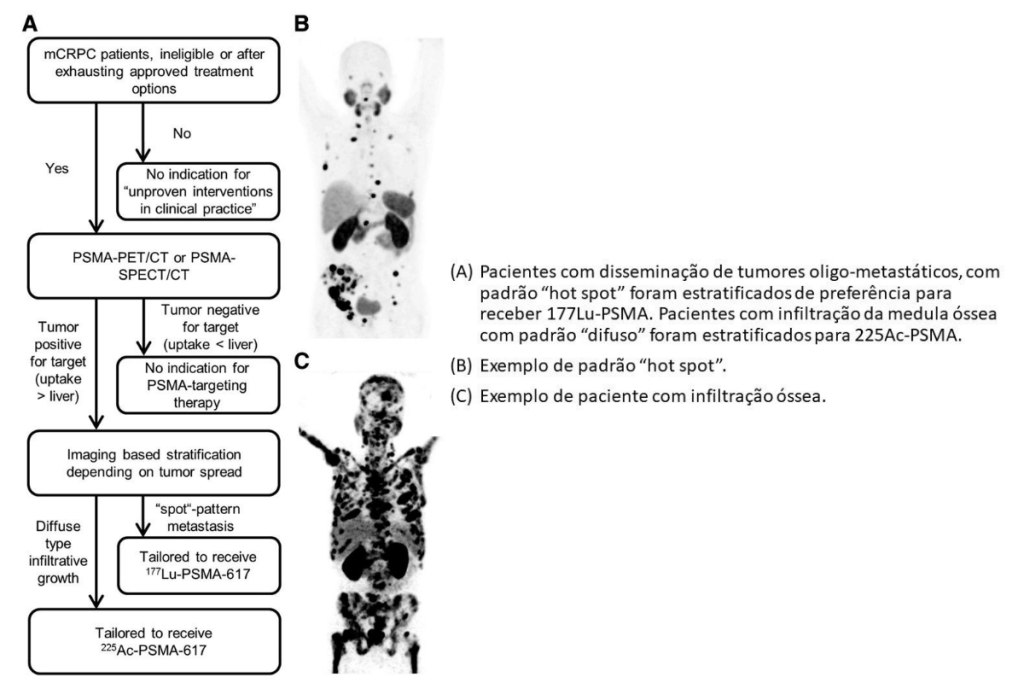
Researchers at the German Cancer Institute suggest that both radiopharmaceuticals would be useful in the treatment of mCRPC depending on the type of metastasis.
By Dr. Alice Viana, Scientific Advisor Oncidium foundation Brazil